A major update to federal preventive guidance for women’s health will make it easier for women to get screened for cervical cancer, including a self-collection option that allows some women to get tested at home instead of going to a doctor for a pelvic exam.
The new option will be covered by private insurance starting in January 2027.
Updated guidance from the Health Resources and Services Administration (HRSA) now recommends that people get a high-risk HPV test, which looks for the types of viruses most likely to cause cervical cancer, every five years for average-risk women ages 30 to 65. as the preferred detection method. This can be done with a sample collected by a doctor or by the patient at home.
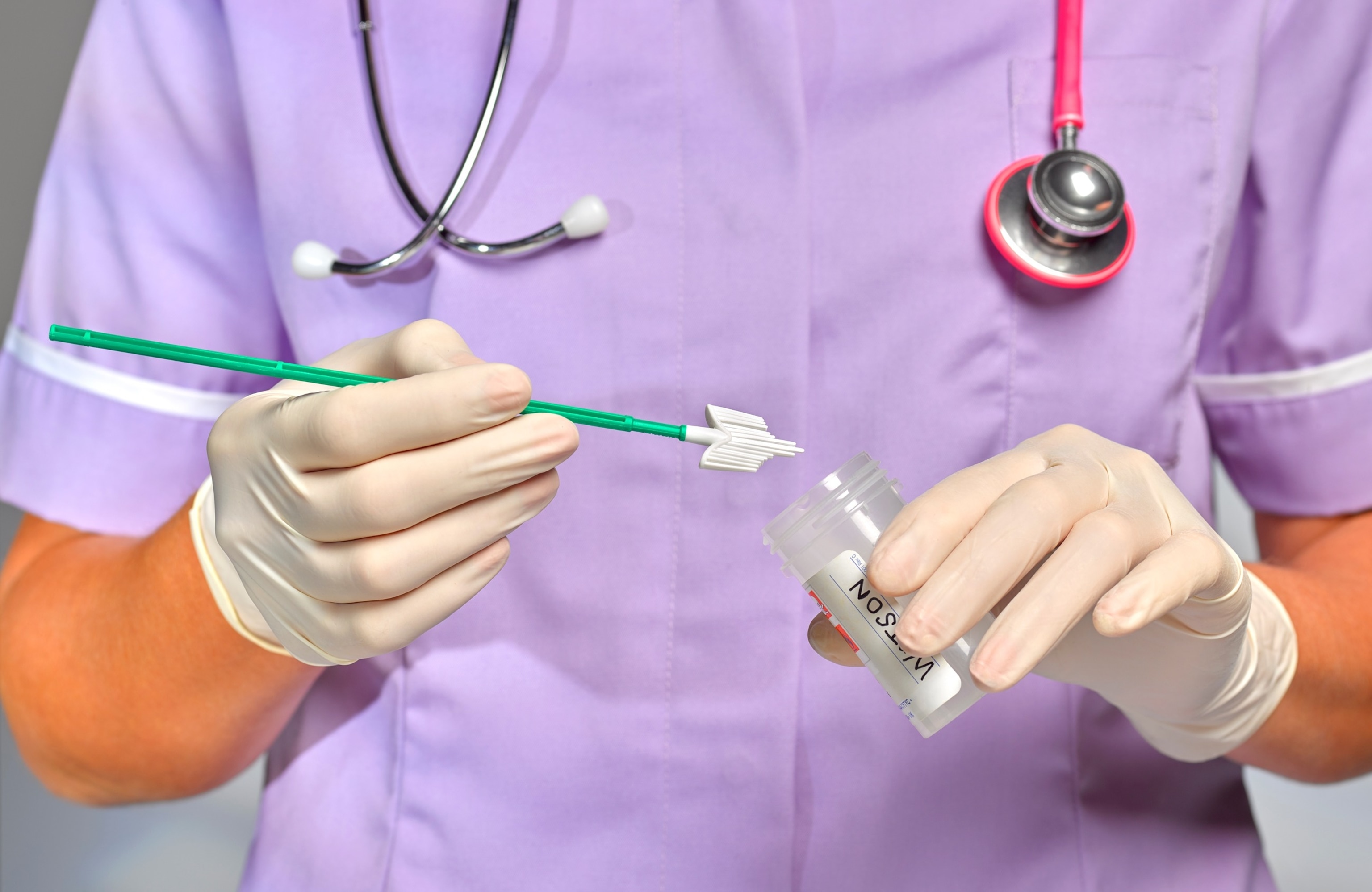
Practice nurse/doctor doing swab test
Peter Dazeley/Getty Images
Women in that age group still have other options: a combination of an HPV and Pap test every five years, or a Pap test alone every three years if HPV testing is not available.
“The addition of self-collection really allows women to make this decision for themselves,” Ann Sheehy, MD, HRSA’s medical director, told ABC News. “We are keeping the Pap test option… this is just an additional option for women.”
For women ages 21 to 29, the recommendations remain the same: Pap tests every three years, which Sheehy said aligns with the available evidence.
“By doing this, we’re going to take out some of those people who have been falling through the cracks and haven’t gotten these tests ahead of time,” HRSA Administrator Tom Engels told ABC News. “And by doing so, we will save lives.”
Engels emphasized that the update is intended to expand testing options, not replace the Pap test. Self-collection aims to remove barriers for women who find in-office screening difficult to schedule, inconvenient or difficult to access, she emphasized.
American Cancer Society (ACS) guidelines In contrast, studies updated in December recommend that cervical cancer screening begin at age 25 and focus on primary HPV testing, including self-collection testing.
“The combination of good evidence of the benefits of self-collection, including increased access to cervical cancer screening, combined with FDA approval, led the ACS and HRSA to include self-collection in their guidelines update,” said Dr. Robert Smith, the American Cancer Society’s senior vice president of Cancer Early Detection Science and author of the organization’s updated guidelines told ABC News.
Cervical cancer screening is often cited as a major public health success. Over the past 50 years, cervical cancer incidence and deaths have decreased by more than 50% in the U.S., according to the American Cancer Society, largely because screening tests can detect precancerous changes early, before patients notice any symptoms.
UNITED STATES – MARCH 09: Bottles of Merck & Co.’s Gardasil cervical cancer vaccine are arranged for a photo at a pharmacy in New York, U.S., on Monday, March 9, 2009. Merck & Co.’s $41.1 billion purchase of Schering-Plough Corp. adds experimental drugs for blood clots, infections and schizophrenia and allows companies to accelerate drug research biotechnological. (Photo by Jb Reed/Bloomberg via Getty Images)
Bloomberg via Getty Images
When cervical cancer is detected early, five-year survival is greater than 90%, according to the Centers for Disease Control and Prevention (CDC). the data suggests. But the HSRA guidance notes that more than half of diagnoses occur beyond the earliest stage, after the disease spreads to other areas of the body. In these last stages, Five-year survival is only about 20%, according to the CDC.
Widespread use of the HPV vaccine is expected to further reduce cervical cancer rates over time, but most of the historic decline occurred before widespread vaccination efforts.
Sheehy said he has seen the consequences when screening is not done and why early detection is important.
“I’ve seen women who didn’t have access to screening and their cancer came at a very advanced stage,” she said. “Most women who have early-stage cervical cancer or precancerous lesions are asymptomatic, and the only way to detect it is through screening.”
The updated guidance aims to address persistent gaps despite decades of progress, she added, noting that about half of women diagnosed with cervical cancer have never been screened or their screening tests are not up to date, and about one in four women in the U.S. are not up to date on screening, according to the CDC.
Cervical cancer cells.
Scientific Photo Library – Cnri/spl/Getty Images
Only FDA-approved tests are recommended for self-collection. FDA expanded approvals for the first time in May 2024 to allow patients to collect samples themselves in a clinical setting. In May 2025, the FDA approved the first cervical cancer screening kit that can be collected at home.
The at-home option is available with a prescription. Exactly how patients access a covered self-collection test may vary by insurer and plan.
“There are some FDA tests that are approved for in-office collection and there is one that is available for at-home collection,” Sheehy noted.
Sheehy and Smith added that a positive HPV result is not a diagnosis of cancer, but it may mean that additional testing is needed.
The updated guidance also aims to reduce costs that can accrue after an abnormal screening test result by clarifying what insurers should cover without cost sharing, including follow-up testing and diagnostic evaluation, such as Pap smears, biopsies, and lab tests, based on individual needs.
A separate HRSA guideline that went into effect Jan. 1 also requires insurance coverage for patient navigation services that help women schedule screenings, address care challenges, and follow up after abnormal results.
“We know that the health care system is incredibly complicated for patients to navigate,” Sheehy said.
Both Engles and Sheehy emphasized how optimistic they are about the potential benefits of expanding access to cervical screening.
“This could really be a game-changer for women,” Sheehy said.
Radhika Malhotra, MD, is an internal medicine and preventive medicine resident at Rutgers Medical School in New Jersey and a member of the ABC News Medical Unit.
ABC News Liz Neporeent contributed to this report.




